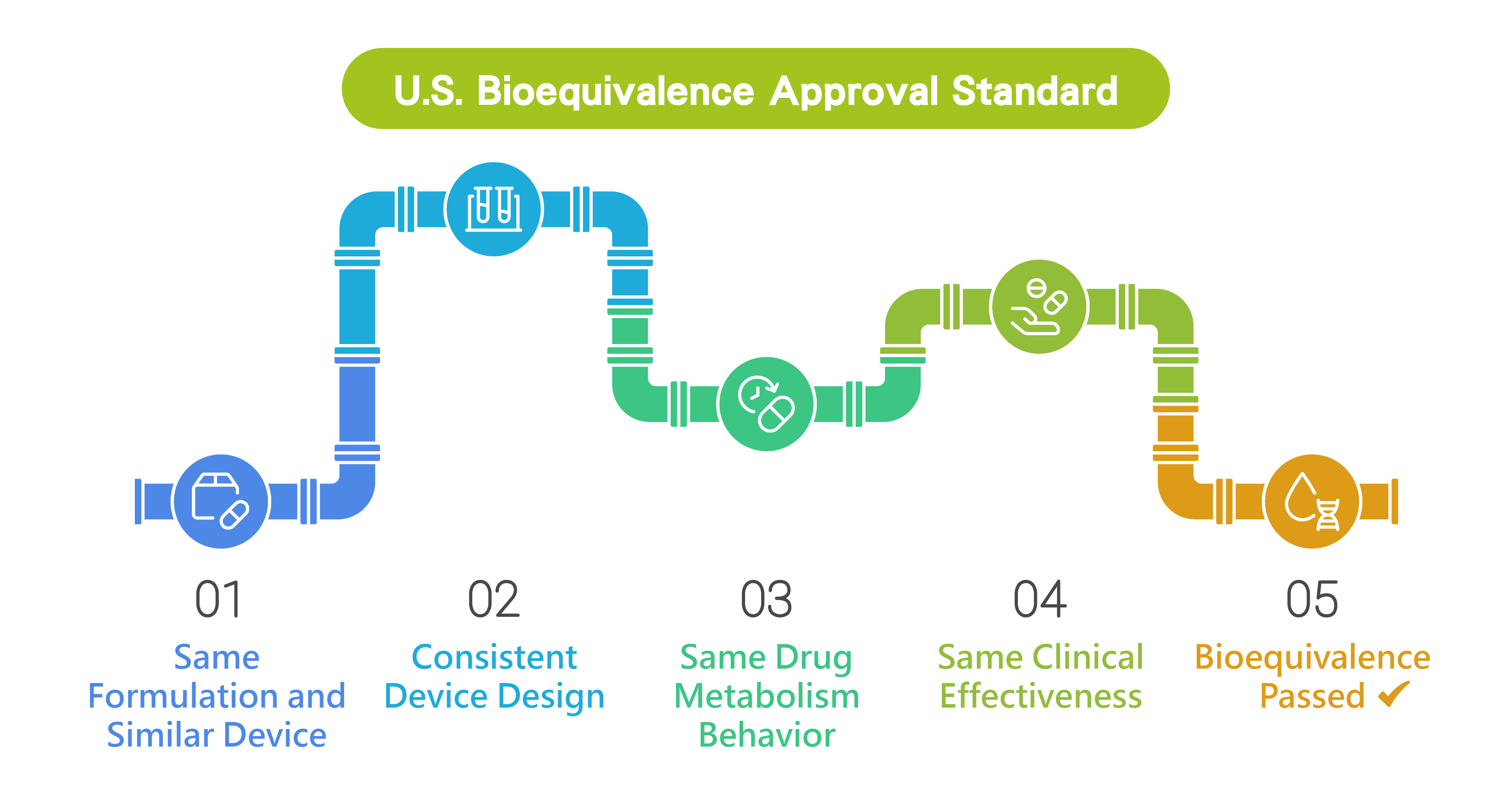
Soft Mist Inhaler
Designed for the treatment of chronic obstructive pulmonary disease (COPD), lower respiratory tract infections, and chronic respiratory diseases, this device enables uniform aerosolization of high value pharmaceuticals and precise delivery to targeted regions of the lungs. Its intuitive operation minimizes the need for hand mouth coordination and inspiratory effort, significantly improving ease of use and patient adherence.
Featuring a mist velocity of 0.8 m/sec and an aerosol duration of 1.5 seconds, the system enhances pulmonary drug with liposomal, biologic, mRNA, protein, antibody drug conjugates (ADC) and small / largemolecule formulations. deposition and bioavailability while reducing systemic side effects. The fully mechanical, propellant free design aligns with international sustainability initiatives and pharmaceutical development requirements.
Drug Development Ready
• 505(b)(2) reformulations and new delivery routes
- ✓Glycopyrronium
- ✓Umeclidinium
- ✓Vilanterol
- ✓Indacaterol
- ✓Inhaled Corticosteroids (ICS)
• 505(b)(1) novel therapies, including biologics and botanical extracts
- ✓ Biologics
- ✓ Pulmonary arterial hypertension therapies
- ✓ Medical cannabinoids
- ✓ Botanical extracts (e.g., eucalyptus leaf essential oil)
Features
+ Semiconductor Process Customized Low Shear Microfluidic Channels
With critical linewidths < 3 µm and precision of 0.3 µm, filtration channels and atomization nozzles are integrated into a silicon/glass chip. The low-shear transport mechanism preserves molecular integrity, enabling highly uniform aerosol generation and improved lung deposition efficiency.
+ Dual Jet Fluid System with 15 µL Precise Metered Dosing (±10%)
A high tension spring (>180 bar) drives a capillary liquid column to collide at a controlled angle, generating a fine, soft mist that ensures consistent, stable, and predictable drug delivery.

+ Velocity 0.8 m/sec with an Aerosol Duration of Up to 1.5 Seconds
Slow, sustained soft mist release, allowing medication to deposit deeper in the lungs. The low resistance nebulization design ensures patient friendly operation and improves treatment adherence.
+ Eco Friendly
No CFC or HFA propellants, no electricity required. A spring powered mechanical system delivers consistent aerosolization, while the replaceable drug cartridge design supports diverse pharmaceutical applications.
+ Replaceable Drug Cartridges
A 4 mL cartridge delivers up to 60 sprays and can be easily replaced after use. Cartridge materials are recyclable, supporting sustainability goals.
+ In Vitro Performance Testing for Inhalation Products
Our Laboratory is accredited to ISO/IEC 17025 and ISO 27424, and complies with EN ISO 27427, EP <0671>, EP <2.9.18>, EP <2.9.44>, USP–NF <601>, and USP–NF <1601> standards. Bioequivalence related testing services.
- ➤ Aerodynamic particle size distribution
- ➤ Fine particle fraction (FPF)
- ➤ Delivered dose uniformity
- ➤ Spray pattern and plume geometry
- ➤ Aerosol duration
- ➤ Cloud velocity
Global IP Strategy & Patent Risk Management
Protects critical manufacturing processes and core technologies while mitigating infringement risks. This strategy extends product life cycles, enables next generation inhaled drug delivery platforms, and establishes long term, defensible competitive advantages.
Accelerate Your Drug Device Launch
.jpg)