简介
通过ISO/IEC 17025认证并符合多国药典,专为吸入性产品(MDI、DPI、SMI、鼻喷剂、喷雾器/雾化器),提供体外效能测试服务(如呼吸模拟、NGI量测等),与校正量测服务。
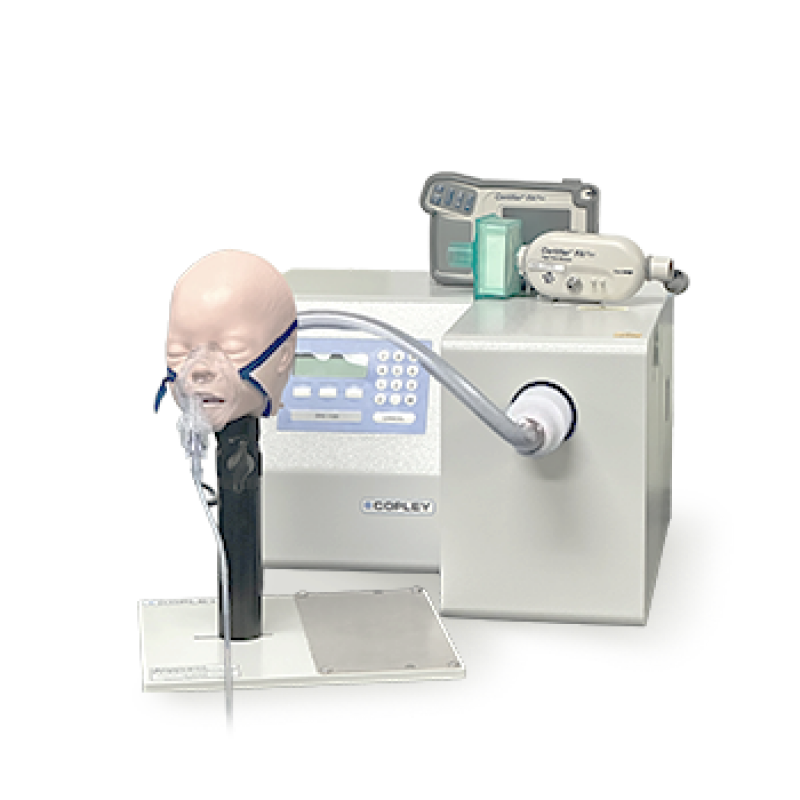
通过ISO/IEC 17025认证并符合多国药典,专为吸入性产品(MDI、DPI、SMI、鼻喷剂、喷雾器/雾化器),提供体外效能测试服务(如呼吸模拟、NGI量测等),与校正量测服务。

我们可以依照客户的需求、国际标准、药典(如:美国药典、欧洲药典、中国药典等),提供客制化的测试分析方案。 我们拥有丰富专业知识的技术专家提供全面的质量保证,协助您确保吸入性产品的质量、安全性和有效性。
我们在国际市场具有丰富的产品注册经验,不仅帮助客户满足监管要求的产品质量和稳定性,并协助产品注册顺利取证。 在每个测试服务完成时,我们会提供详细的测试报告。
我们在吸入性产品拥有数十年的丰富经验,可以帮助您成功地将吸入产品推向市场。 在产品开发的所有阶段为您提供支持及专业的知识。
美国药典USP <1601>
欧洲药典 EP <2.9.44>
国际标准 ISO 27427
英国/国际标准 EN ISO 27427
英国标准 EN 13544-1